Volume 3, Year 2016 - Pages 1-6
DOI: TBD
Evaluation of an Indigenous Dunaliella Strain for β-Caroten and Neutral Lipid Production as a Response to Cobalt Deprivation
Zeynep Elibol Çakmak1,2, Turgay Çakmak2
1Kırıkkale University, Department of Biology
Yahsihan, Kırıkkale, Turkey
zelibolcakmak@gmail.com
2Istanbul Medeniyet University, Department of Molecular Biology and Genetics
İstanbul, Turkey
turgay.cakmak@medeniyet.edu.tr
Abstract - Microalgae produce a wide range of metabolites to serve for several biotechnological applications. Sustainable production of neutral lipids and carotenoids from microalgae are of special importance in algal biotechnology industry. In this study, triacylglycerol (TAG) and β-caroten production of an indigenous Dunaliella strain was investigated as a response to Cobalt (Co) deprivation during 30 days of incubation period. Based on genomic analysis, the strain was identified as Dunaliella tertiolecta IMCC-37. Long term Co deprivation did not cause considerable change in growth while photosynthesis activity increased as corroborated with increase in Chlorophyll-a and Chl-a/b ratio. Induction of TAG production of over 27% was supported by 11% increase in neutral lipid content of Co-deprived D.tertiolecta IMCC-37. A simultaneous increase in β-caroten level with a value of 14% was correlated with significant increase of carbohydrate level even if there was no change in total carotenoid production of Co-deprived strain. Results show that Co deprivation may be used as a potential tool for increasing both TAG and β-caroten production by D.tertiolecta.
Keywords: Biodiesel, Cobalt, Dunaliella tertiolecta, Halotolerant microalgae, Lake Meke.
© Copyright 2016 Authors - This is an Open Access article published under the Creative Commons Attribution License terms. Unrestricted use, distribution, and reproduction in any medium are permitted, provided the original work is properly cited.
Date Received: 2015-08-20
Date Accepted: 2016-06-21
Date Published: 2016-07-08
1. Introduction
Microalgae are single-celled, ubiquitous, primary photosynthetic microorganisms. Being a fundamental step of the nutrient chain, microalgae offer a wide range of metabolites which are of biotechnological importance [1]. Microalgae are currently utilized as fertilizer in agriculture, as food supplement in food industry, and a dynamic solution for bioremediation; nevertheless, they are most pronounced for production of several primary and secondary metabolites including lipids as biodiesel feedstock and carotenoids as food supplement [2]. Amongst microalgae, Dunaliella strains are mostly pronounced with their halotolerant and competitive nature [3]. The genus Dunaliella includes around 30 species of which 25 are found in saline water. Several type of microalgae strains from Dunaliella genus are distinguished with their ability to produce β-caroten when they are grown under high salt conditions [4]. On the other hand, strain Dunaliella tertiolecta was stated to have potential use of biodiesel feedstock production with a reported oil yield of 36-42% [5].
Microalgae adjust their cellular redox status dynamically in response to fluctuations in their environments, nutrient deprivation in particular [6]. Remarkable studies have been reported on impact of several stress factors on neutral lipid content of D.tertiolecta. Tang et al. [7] evaluated influence of CO2 concentration, photoperiod, light source and intensity on growth and lipid production of D.tertiolecta. They reported that LED or fluorescent light sources do not effect growth or lipid content while 2-6% CO2 levels provide highest growth. Chen et al. [8] reported that Iron (Fe), Manganese (Mn), Molybdenium (Mo), Zinc (Zn) and finally Cobalt (Co) are required for growth of D.tertiolecta, and deficiency of Fe or Co cause decreases in growth and short term induction of neutral lipid production. They reported that Co and Fe deprivation causes a significant increase in neutral lipid content on 3rd day; however, in the same study decreased neutral lipid level was reported on 7th day of Co or Fe deprivation. In the same study, rapid and consistent accumulation of neutral lipids followed by a rapid decrease in growth was reported as a response to Nitrogen (N) deprivation during 5 days of incubation period. N-deprivation induced neutral lipid production by microalgae have been reported by several researchers so far; however, rapid decrease of cell growth remains a big penalty [9,10.]. Nevertheless, N and Fe overabundance were reported to cause significant increases in both growth and lipid productivity in D.tertiolecta [11].
β- carotene is an essential nutrient and has high demand in the market as a natural food colourant and a health supplement [12]. Amongst Dunaliella strains, Dunaliella salina is most pronounced for its high β-carotene content especially when it is exposed to high salts stress [12]. On the other hand, increased level of accessory photoprotective pigments (α-carotene and β-carotene) were reported when D.tertiolecta was grown under Phosphorus (P) or N-deprived conditions [13]. To the best of our knowledge, there is so far no study evaluating carotenoid and neutral lipid production efficiency of D.tertiolecta in response to a trace element, Co, deprivation. In this study, we report impact of Co deprivation on neutral lipid and β-caroten production by an indigenous strain D.tertiolecta IMCC-37.
2. Methods
Dunaliella tertiol ecta IMCC-37 was isolated from a volcanic saline lake, Lake Meke, located in Konya Province with the coordinates of 37o 40’ 32’’- 37o 41’ 33’’ north latitude and 33o 38’ 36’’ - 33o 38’ 61’’ east longitude. The isolated strain was identified based on genomic information. Genomic DNA was extracted from algal species following phenol-chloroform method on a pellet obtained by centrifugation of 10 mL of algal culture at the late-log phase [14]. DNA amplification from genomic DNA containing a partial 18S ribosomal RNA region was performed by PCR using the following primers: Forward: 5‘-ATTGGAGGGCAAGTCTGGT-3‘ and Reverse: 5‘- ACTAAGAACGGCCATGCAC-3‘. Same primers were used for Sanger sequencing. Nucleotide sequences were analysed on NCBI database and finally BLAST results were used for identification of the strain.
D.tertiolecta IMCC-37 strain was grown in 50ml medium in 100ml flasks under continuous light intensity of 250 µE/(m2/s) in a temperature-controlled orbital shaker with a 120rpm speed under 28oC temperature. Modified Jhonsons medium [15] with 20% salt concentration was used as control and CoCl2 was not included in the growth medium for Co deprivation. Microalgae were cultivated under defined conditions for 30 days and growth was monitored. Growth was recorded by measuring absorbance of the culture at 680nm. Oxygen production and consumption efficiency, chlorophyll, carotenoid contents, neutral lipid, triacylglycerol (TAG) and carbohydrate levels were measured over 15 days of incubation period which is defined as exponential growth phase for the strain D.tertiolecta IMCC-37.
Net photosynthesis and respiration rates of control and Co-deprived D.tertiolecta were measured as a function of oxygen production or consumption levels using a Clark-type oxygen electrode system (Hansatech Oxytherm, Hansatech Ins. Ltd., Norfolk, U.K.). 2 ml of cell culture with adjusted absorbance value of (A680 = 1.0) with respective solutions was inoculated into the reaction vessels and continuously stirred at 25°C. Cells were adapted to dark conditions with monitoring of oxygen consumption for 6 min. The rate of oxygen consumption was measured over 5 min of incubation. Then the oxygen production level was calculated, through the illumination of the vessel by a projector lamp with an intensity of 480 µE/(m2/s).
Chlorophyll and carotenoid content of cells were measured as described before [16]. HPLC analysis of β-carotene was performed as suggested in the literature [17]. Neutral lipid staining was performed using Nile Red as described before [18]. Triacylglycerol and carbohydrate levels were measured by Fourrier transform infrared (FT-IR) spectroscopy measurement. For FT-IR measurement, cells were concentrated on a 96 well silicon microplate and oven-dried for 45 min to form homogeneous thin films [19]. FTIR spectra were recorded using a Nicolet 6700 Research FT-IR Spectrometer (Thermo Scientific). The bands were assigned to specific molecular groups on the basis of biochemical standards and published studies as previously described [20]. FTIR peak values were of particular interest which were attributed to ester group (C=O) vibration of triglycerides (1744 cm-1), carbohydrate bands (1200–950 cm-1) and amide I absorption (1652 cm-1).
The final data of each experimental group in this article are mean values represented by at least six replicate samples. Standard errors and t-tests (two tails, pair type) with significance criteria of 0.05, 0.01, or 0.001 were used to assess significance.
3. Results and Discussion
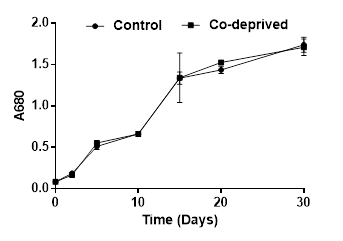
Being an integral component of vitamin B12, Co is stated to play a biolimiting role in the oceans [21]. Cobalt has been shown to be an important micronutrient for Dunaliella tertiolecta growth [8]. Cobalt deprivation was reported to cause remarkable decreases in growth starting from third day of deprivation [8]. However, in this study, Co deprivation did not cause any significant change in growth during 30 days of incubation period (Figure 1). This difference might be due to different growth solutions used in reported [8] and the current study. They used Erdschreiber’s medium enriched with soil water; however, we used modified Jhonson’s medium without soil water. Moreover, they grew microalgae on 96-well microplates and growth temperature was 32oC while it was 28oC in our study and we incubated microalgae in 250ml flasks with continuous shaking and illumination.
Photosynthetic efficiency of microalgae is predominantly reflected by the chlorophyll and carotenoid levels. Microalgae need to keep their chlorophyll and carotenoid contents in a balance for an efficient utilization of carbon sources [22]. Our results showed that Oxygen evolution activity of Co-deprived D.tertiolecta cells increased as supported by increase in Chlorophyll-a and Chl-a/b ratio. (Figure 2). An increase in the Chlorophyll a/b ratio reflects changes in the size of the antenna complex and PSII/PSI [23].
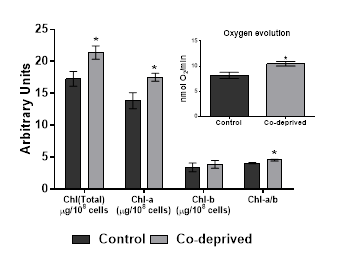
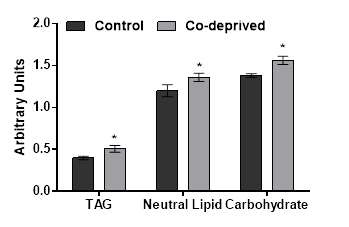
Slight increases in oxygen evolution activity and chlorophyll levels in D.tertiolecta refers to increased anabolic activity. Increased anabolic activity would either lead increased cell growth or production of proteins, carbohydrates or lipid based biomolecules. There was no significant difference in growth (Figure1). However, nutrient deprivation often induce alteration of cellular macromolecular compositon [24,25]. Thus, neutral lipid and carbohydrate levels of 15 days old controlled or Co-deprived D.tertiolecta cells were analysed. Fourier transform infrared (FTIR) spectroscopy offers sensitive and rapid measurement of macromolecular composition of a microalgal cell [25]. Triacylglycerol levels increased over 27% as supported by 11% increase in neutral lipid content (Figure 3). Short term increase in neutral lipid content of D.tertiolecta in response to Co deprivation was reported before [8]. However, increase in neutral lipid content on 3rd day was followed with significant decrease on 7th day of Co deprivation in the same study. They used only Nile Red staining to determine neutral lipid levels in D.tertiolecta. Including D.tertiolecta, most microalgae accumulates cytoplasmic lipid droplets in response to a stress factor. Lipid droplets are composed of a neutral lipid core consisting mainly of TAGs. Thus, increase in neutral lipid content in this study is supported by significant increase of TAG level in Co-deprived D.tertiolecta. Moreover, carbohydrate level increased over 13% as a response to 15 days Co-deprivation (Figure 3). Increased carbohydrate levels would refer to production of different saccharides as a response to fluctuations in photosynthesis and respiration efficiency of Co-deprived cells.
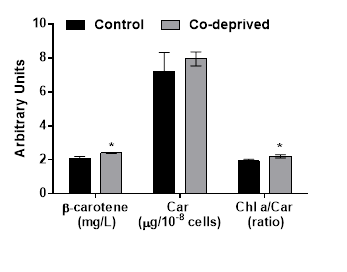
Halophilic Dunaliella strains are most pronounced for their ability to produce β-caroten under stress conditions [12]. Even if there was no significant change in total carotenoid production, β-caroten production increased over 14% at the end of 15days of incubation period (Figure 4). Increase in β-caroten content of D.tertiolecta in response to N or P deprivation, were reported before [13]. Here we report significant increase of β-caroten in D.tertiolecta in response to Co-deprivation. β-caroten is the most common accessory photoprotective pigments in Dunaliella strains [17]. Increase in β-caroten production is supported by increased oxygen evolution efficiency of D. tertiolecta in this study (Figure 2). Increased Chlorophyll a/Carotenoid level would indicate increase in both photosyhtnesis activity and weak oxidative stress in response to Co-deprivation. Increase in carotenoid content of algal cells was previously reported as part of a defense mechanism against photo-damage [6,26].
In this study, Co deprivation was found to induce neutral lipid and β-carotene production of D.tertiolecta as measured on exponential growth phase. Production of β-carotene by D.tertiolecta, as well as other halotolerant Dunaliella strains, was reported to increase in response to a stress factor such as high salt conditions [17]. On the other hand, Co deprivation was shown to induce a short term lipid production while causing decrease in growth of D.tertiolecta [8]. This study shows that, Co deprivation induce β-carotene and neutral lipid production of D.tertiolecta and it does not cause any significant change in growth. Increase in photosynthesis activity and photosynthetic pigment levels refer to increased metabolism which is not reflected by a change in growth but by increase in metabolites such as lipids and carbohydrates.
4. Conclusion
A halotolerant microalga, D.tertiolecta is a remarkable candidate for biodiesel feedstock, production. This species is not only pronounced for its high lipid content but β-carotene content of this species is also at a considerable level. Thus, we define D.tertiolecta as a potential candidate for production of TAG and β-carotene. In this study, Co deprivation did not cause significant change in growth while significant increase of TAG and β-carotene content were observed. A major conclusion of this study is that Co deprivation can be used as a potential tool for increasing both TAG and β-caroten production by D.tertiolecta.
Acknowledgements
This study was supported by a grant from the Scientific and Technological Research Council of Turkey (Project# 112Y029).
References
[1] M. J. Griffiths, R. G. Dicks, C. Richardson and S. T. L. Harrison, “Advantages and challenges of microalgae as a source of oil for biodiesel,” in Biodiesel- Feedstocks and Processing Technologies, M. Stoytcheva, Ed. New York, InTech, 2011, pp. 177-200. View Article
[2] Y. Chisti, “Biodiesel from microalgae,” Biotechnol Adv., vol. 25, pp. 294-306, 2007. View Article
[3] A. S. Cifuentes, M. A. Gonzalez, I. Inostroza and A. Aguilera, “Reappraisal of physiological attributes of nine strains of Dunaliella (Chlorophyceae): growth and pigment content across a salinity gradient,” J. Phycol., vol. 37, pp. 334-344, 2001. View Article
[4] P. Spolaore, C. Joannis-Cassan, E. Duran and A. Isambert, “Commercial applications of microalgae,” J. Biosci. Bioeng., vol. 101, pp. 87-96, 2006. View Article
[5] K. Tsukahara and S. Sawayama, “Liquid Fuel Production using Microalgae,” J Japan Petrol Inst., vol. 48, pp. 251-259, 2011. View Article
[6] Z. Elibol Çakmak, T. T. Olmez, T. Çakmak, Y. Menemen and T. Tekinay, “Antioxidant response of Chlamydomonas reinhardtii grown under different element regimes,” Phycol. Res., vol. 63, pp. 202-211. View Article
[7] H. Tang, N. Abunasser, M. E. D. Garcia, M. Chen, K. Y. Simon and S. O. Salley, “Potential of microalgae oil from Dunaliella tertiolecta as a feedstock for biodiesel,” Appl. Energy, vol. 88, pp. 3324-3330, 2011. View Article
[8] M. Chen, H. Tang, H. Ma, T. C. Holland, K. Y Simon and S. O. Salley, “Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta,” Bioresource Technol., vol. 102, pp. 1649-1655, 2011. View Article
[9] Z. Elibol Çakmak, T. T. Olmez, T. Çakmak, Y. Menemen and T. Tekinay, “Induction of triacylglycerol production in Chlamydomonas reinhardtii: Comparative analysis of different element regimes,” Bioresource Technol., vol. 155, pp. 379-387, 2014. View Article
[10] X. Wu, R. Ruan, Z. Du and Y. Liu, “Current status and prospects of biodiesel production from microalgae,” Energies, vol. 5, pp. 2667-2682, 2012. View Article
[11] T. M. Mata, R. Almeidab and N. S. Caetona, “Effect of the culture nutrients on the biomass and lipid productivities of microalgae Dunaliella tertiolecta,” Chem Eng Trans., vol. 32, pp. 973-978, 2013. View Article
[12] R. Raja, S. Hemaiswarya and R. Rengasamy, “Exploitation of Dunaliella for β-carotene production,” Appl Microbiol Biotechnol., vol. 74, pp. 517-523, 2007. View Article
[13] R. J. Geider, L. Hugh, M. G. Lisa and M. L. Mckay, “Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorous limitation,” European J Phycol., vol. 33, pp. 315-332, 1998. View Article
[14] P. Chomczynski and N. Sacchi, “Single-step method of RNA isolation by acid guanidium thiocyanate phenol chloroform extraction,” Anal Biochem., vol. 162, pp. 156-159, 1987. View Article
[15] M. A. Borowitzka, “Algal growth media and sources of cultures,” in Microalgal Biotechnology, Cambridge, UK: Cambridge University Press, 1988, pp. 456-465.
[16] S. W. Jeffrey and G. F. Humphrey, “New spectrophotometric equations for determining chlorophylls a, b, c1, and c2 in higher plants, algae, and natural phytoplankton,” Biochem Physio Pfl., vol. 167, pp. 191-194, 1975. View Article
[17] M. R. Fazeli, H. Tofighi, N. Samadi and H. Jamalifar, “Effects of salinity on β-carotene production by Dunaliella tertiolecta DCCBC26 isolated from the Urmia salt lake, north of Iran,” Bioresource Technol., vol. 97, pp. 2543-2546, 2006. View Article
[18] D. Elsey, D. Jameson, B. Raleigh and M. J. Cooney, “Fluorescent measurement of microalgal neutral lipids,” J Microbiol Meth., vol. 68, pp. 639-642, 2007. View Article
[19] A. P. Dean, D. C. Sigee, B. Estrada and J. K. Pittman, “Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae,” Bioresource Technol., vol. 101, pp. 4499-4507, 2010. View Article
[20] Z. Movasaghi, S. Rehman and I. Rehman, “Fourier transform infrared (FTIR) spectroscopy of biological tissues,” Appl Spectrosc Rev., vol. 43, pp. 134-179, 2008. View Article
[21] K. W. Bruland, “Trace elements in seawater,” in Chemical oceanography, K. W. Brulan Ed. London: Academic Press, 1983, vol. 8, pp. 157-220. View Article
[22] L. Zhang, T. Happe and A. Melis, “Biochemical and morphological characterization of sulfur-deprived and H-2-producing Chlamydomonas reinhardtii (green alga),” Planta, vol. 214, pp. 552-561, 2002. View Article
[23] A. Melis, “Dynamics of photosynthetic membrane composition and function,” Biochim. Biophys. Acta, vol. 058, pp. 87-106, 1991. View Article
[24] Y. Gao, M. Yang and C. Wang, “Nutrient deprivation enhances lipid content in marine microalgae,” Bioresource Technol., vol. 147, pp. 484-491, 2013 View Article
[25] T. Cakmak, P. Angun, Y. E. Demiray, A. D. Ozkan, Z. Elibol and T. Tekinay, “Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii,” Biotech Bioeng., vol. 109, pp. 1947-1957, 2012. View Article
[26] H. K. Ledford and K. K. Niyogi, “Singlet oxygen and photooxidative stress management in plants and algae,” Plant Cell Environ., vol. 28, pp. 1037-1045, 2005. View Article

